Nūhou MaikaʻiʻAʻole maopopo iaʻu!IVDR CECpalapala hōʻoia no ACCUGENCE®Pnā huahana
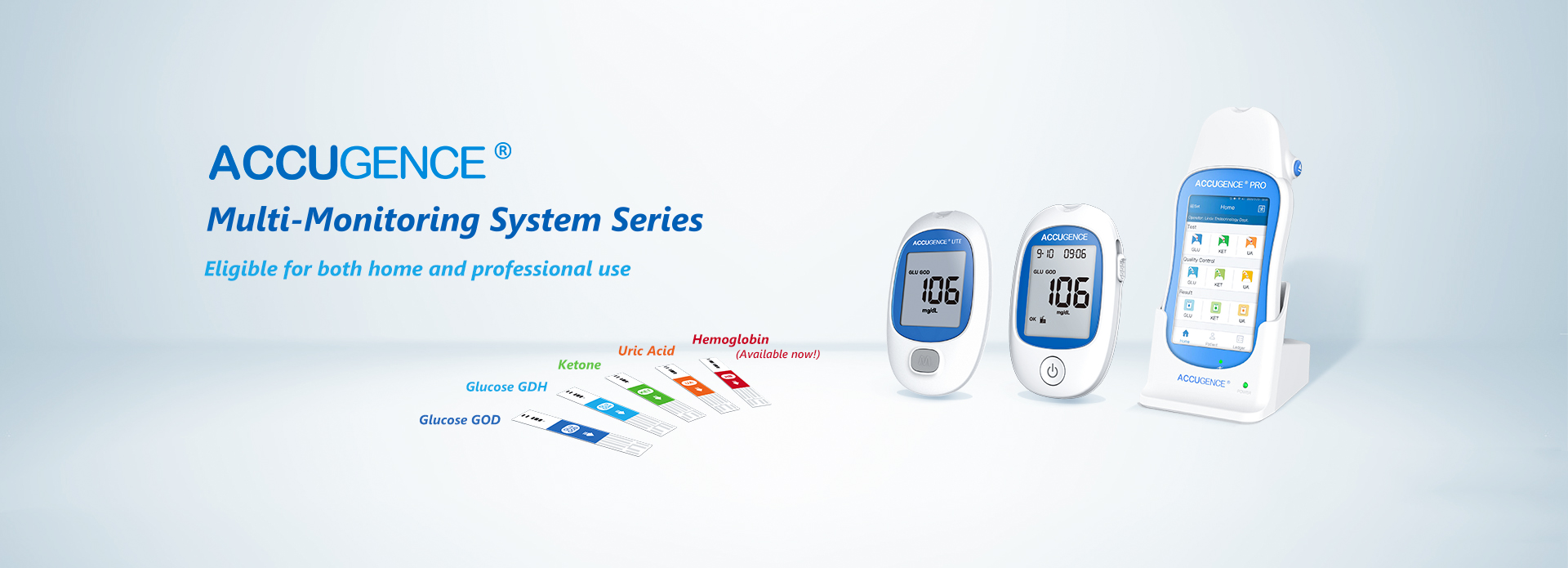
Ma ka lā 11 o ʻOkakopa, ua hoʻolaha ʻia ka ACCUGENCE Multi-Monitoring System ACCUGENCE® Multi-Monitoring Meter (ACCUGENCE Blood Glucose, Ketone a me Uric Acid Analysis System, me ka Meter PM900, Blood Glucose Strips SM211, Blood Ketone Strips SM311, Uric Acid Strips SM411, a pēlā aku.)ua hala i ka palapala hōʻoia Papa C o IVDR.
Ma ka loaʻa ʻana o ka palapala hōʻoia IVDR CE i hoʻopuka ʻia e TÜV SÜD, ke kino i hoʻolaha ʻia o ka ʻAhahui ʻEulopa, he ʻanuʻu koʻikoʻi ia i ka holomua o ACCUGENCE®, a he hōʻailona ia i kahi holomua nui i ke kaʻina hana o ka ʻimi ʻana i ka mākeke ma waho o ka ʻāina o e-LinkCare.
E pili ana iā IVDR
ʻO ke EU In Vitro Diagnostic Medical Devices Regulation (IVDR), i hoʻomaka i ka lā 25 o Mei, 2017 a hoʻokō ʻia ma ka lā 26 o Mei, 2022, he mau koi piha a koʻikoʻi hoʻi no ka loiloi loea, ka loiloi lapaʻau, a me ka nānā ʻana i ka mākeke o nā mea lapaʻau diagnostic in vitro e hōʻoia i ka palekana, ka pono, a me ka maikaʻi o nā huahana.
Wahi a nā lula o nā hāmeʻa lapaʻau diagnostic in vitro a ka EU, ʻo ka loaʻa ʻana o ka palapala hōʻoia IVDR CE he mea pono ia no ke komo ʻana o ka huahana i ka mākeke EU, ʻo ia hoʻi, ua loaʻa i ka huahana kahi "visa" e komo ai i ka mākeke ʻEulopa.
ʻO ka hiki i kā mākou huahana ke loaʻa i ka palapala hōʻoia IVDR CE e hōʻike ana i kā mākou ACCUGENCE®Ua hoʻokō ka ʻōnaehana Multi-Monitoring i nā koi kūlana kiʻekiʻe o ka mākeke ʻEulopa ma ke ʻano o ka maikaʻi o ka huahana, ka palekana a me ka pono, a me ka pae loea, apū kekahiua hōʻea ka pae kaohi maikaʻi i nā kūlana honua.
Ka manawa hoʻouna: ʻOkakopa-25-2024